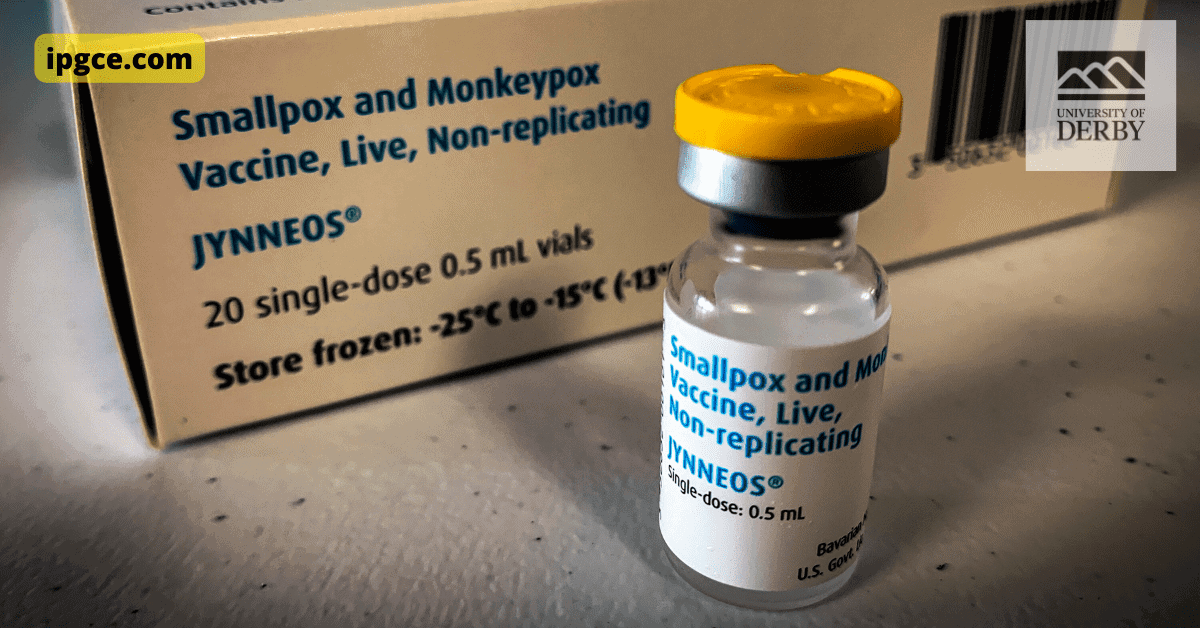The only medicine offered to deal with monkeypox is so difficult to access that simply a portion of the almost 7,000 patients in the United States has been provided it.
.
Wellness officials have actually marked tecovirimat, likewise called Tpoxx, an “investigational medicine,” which implies it can not be released from the tactical nationwide stockpile without a collection of intricate governmental steps. Many physicians do not have the time or sources to fill out the called for 27-page application or to give in-depth patient details.
.
Professionals say it does not have to be this method: There is no legislation avoiding health and wellness officials from making tecovirimat more extensively available. It commonly creates a high temperature and rash and also can be deadly in some instances.
In 2018, there were greater than 7,000 monkeypox instances in the United States, according to the Centers for Disease Control and also Prevention. Just eight people were treated with tecovirimat.
.
The Food and also Drug Administration does not yet authorize Tecovirimat, however it has been assigned as an “investigational brand-new drug” by the firm. That classification permits people to receive the medicine if they satisfy specific standards, such as being signed up in a clinical trial or becoming part of a thoughtful usage program.
.
A lot of medical professionals are strange with the procedure for accessing tecovirimat, as well as the FDA has actually not made it very easy to obtain the medicine.
.
To obtain tecovirimat from the tactical national accumulation, doctors have to complete a 27-page application as well as provide detailed individual info, consisting of laboratory results as well as contact details for the client’s healthcare team.
The FDA states it is servicing improving the procedure, however, for currently, the administration stays a
obstacle for many medical professionals.” The process is burdensome, and also it’s created for research settings, except clinical care,” stated Dr William Schaffner, an infectious condition specialist at Vanderbilt University Medical Center.
.
Sometimes, people may get tecovirimat via a caring use program, which permits drugs to be used in life-threatening scenarios prior to the FDA approves them. Those programs are additionally challenging to browse, as well as most patients will not be eligible for them.
.
For now, the very best expect monkeypox people is that the FDA will certainly approve tecovirimat for basic use. However that could take years, and also in the meanwhile, patients will continue to endure.
.
” It’s a pity since this medication might help lots of people,” Schaffner said. “But now, it’s just out of reach.”.
.
How should the U.S. federal government make tecovirimat extra widely offered to patients? Should the FDA authorize tecovirimat for basic use? Do you think the FDA should improve the process for accessing tecovirimat?
For more news on worldwide education, politics, socio-economics, and so on, follow us on IPGCE and WeChat.
Wechat Code:.
Professionals say it doesn’t have to be this way: There is no legislation preventing health and wellness officials from making tecovirimat much more commonly offered. Exactly how should the U.S. government make tecovirimat extra widely offered to clients? Should the FDA approve tecovirimat for basic use? What are your thoughts on the current process for accessing tecovirimat? Do you believe the FDA should streamline the process for accessing tecovirimat?
Meet Our Successful Graduates: Learn how our courses have propelled graduates into rewarding
careers. Explore their success stories here!
Discover More About Your Future: Interested in advancing your teaching career? Explore our
IPGCE, MA, and QTS courses today!

Explore Our Courses: Ready to take the next
step in your education journey? View our
comprehensive course offerings now!